Institute of Immunology and Infection Research,
School of Biological Sciences,
The University of Edinburgh,
United Kingdom.
Host-parasite interactions and life-threatening malaria.
Individual variation in disease symptoms is one of the unexplained features of malaria. Although approximately half a million children die from malaria every year, many more suffer milder, non-life-threatening forms of the disease. Despite decades of research, it is unclear to what extent this individual variation in disease severity is due to parasite factors, host factors, or a combination of the two. Our main interest lies in investigating the interplay between malaria parasite virulence factors (adhesion molecules) and human genetics (malaria resistance genes) that explain why some children, but not others, develop life-threatening malaria. The importance of this work is that by gaining a greater understanding of the processes leading to severe disease, it may be possible to develop drugs or vaccines to reduce death and disability from malaria.
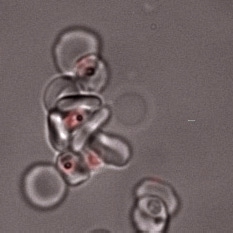
The main focus of the lab is investigating how Plasmodium falciparum infected red blood cells bind to human cells to cause disease. We are studying three major types of host-parasite interaction: rosetting of infected red cells with uninfected red cells; adhesion of infected red cells to Human Brain Endothelial Cells (HBEC-binding); and platelet-mediated clumping of infected red cells.
Rosetting is a parasite adhesion phenotype strongly associated with life-threatening malaria in African children. We aim to understand the molecular mechanisms of rosette formation in order to develop “rosette-busting” drugs or vaccines. In particular, we are studying the role of var genes encoding the infected erythrocyte variant surface protein PfEMP1 in rosetting, and the potential for interventions against PfEMP1 to inhibit rosetting.
Recently, we have also begun to focus on HBEC-binding as an in vitro model for cerebral malaria, investigating the role of PfEMP1 variants as parasite adhesion molecules responsible for HBEC-binding, and the potential for anti-PfEMP1 drugs and vaccines to treat or prevent cerebral malaria. On the host side, we continue to study the red blood cell complement regulatory protein Complement Receptor One that plays a crucial role in rosette formation, and to investigate novel host cell receptors. We are also investigating the role of human IgM natural antibodies in malaria parasite adhesion.
In all our projects we aim to combine detailed in vitro investigation of laboratory culture-adapted parasite lines with studies on fresh clinical isolates from malaria patients in sub-Saharan Africa, in order to establish the relevance of our findings to malaria in the real world. We have recently started to use CRISPR-Cas9 genome editing on both parasite and host cells. Going forward, this new technology will revolutionize our ability to understand the molecular mechanisms of host-parasite interaction in malaria.